Communication Tools
Access templates to inform healthcare teams and patients of important policy changes, vaccine recommendations, and standards of care.
Resources For Health Systems
to your clipboard Templated Email to Healthcare Team #1
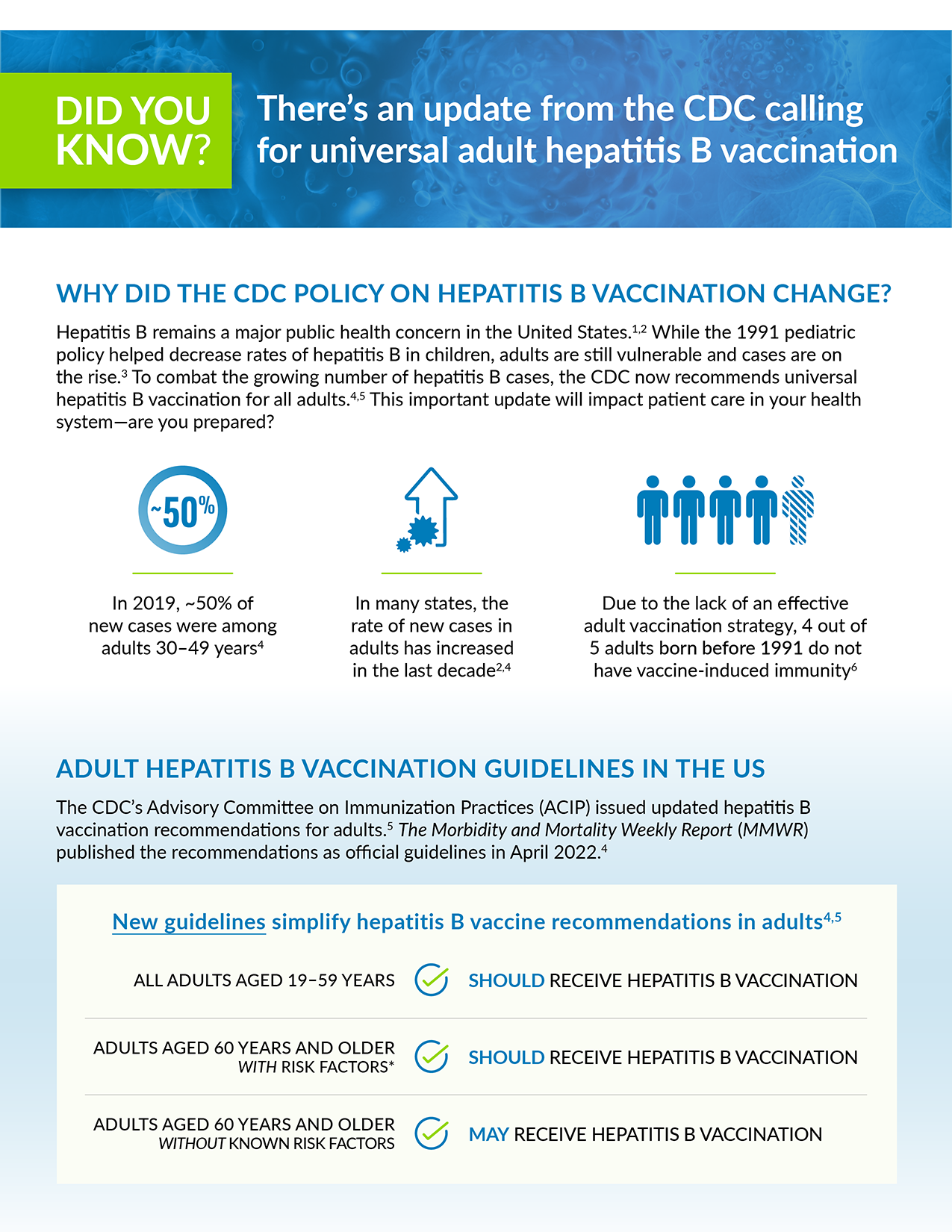
Dear Team,
In April 2022, the Centers for Disease Control and Prevention (CDC) published a universal recommendation to vaccinate all adults (19–59 years) against hepatitis B.1*
Hepatitis B remains a major public health concern in the United States.2,3 While the 1991 pediatric policy helped decrease rates of hepatitis B in children, adults are still vulnerable and cases are on the rise.4 To combat the growing number of hepatitis B cases, the CDC now recommends universal hepatitis B vaccination in eligible adults.1,5
In order to increase vaccination rates, please begin identifying all adults recommended for hepatitis B vaccination in accordance with the CDC’s updated universal policy.1* We will also be updating our EHR system to comply with the policy and help you and your team identify adult patients recommended for vaccination against hepatitis B.
Sincerely,
[XXX]
*The Centers for Disease Control and Prevention (CDC) recommends hepatitis B vaccination for all adults aged 19 through 59 years and adults 60 years or older with risk factors for hepatitis B. Adults who are 60 years or older without known risk factors may also receive vaccination.1
References: 1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483. 2. Hepatitis B Foundation. Do you know your hepatitis facts from fiction? May 7, 2014. Accessed January 31, 2023. https://www.hepb.org/blog/do-you-know-your-hepatitis-facts-from-fiction/ 3. Hepatitis B Foundation. Hepatitis B facts and figures. Accessed January 31, 2023. https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/facts-and-figures/ 4. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR-13):1-25. 5. Murthy N, Wodi AP, Bernstein H, McNally V, Cineas S, Ault K. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:229–233.
US-23-00-00020 | March 2023
to your clipboard Templated Email to Healthcare Team #2
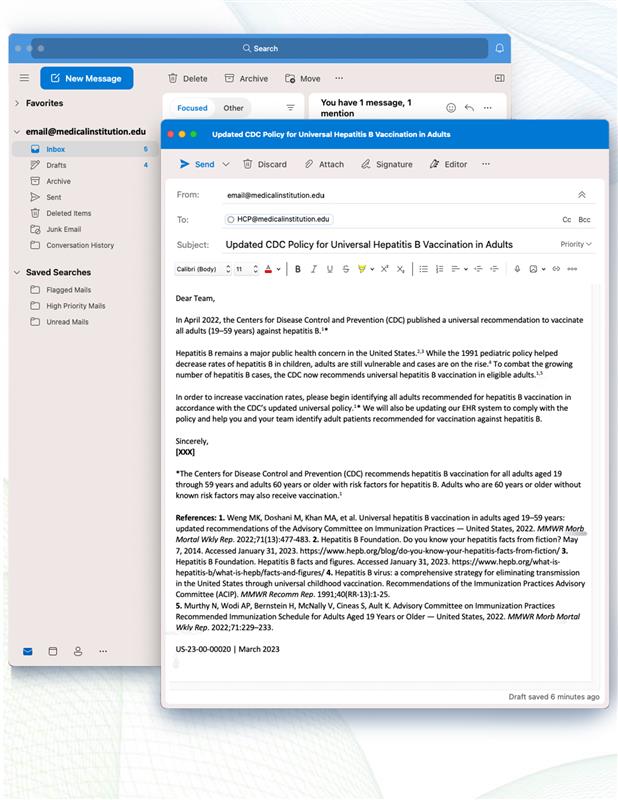
Dear Team,
As you know, the CDC has updated its policy to recommend universal hepatitis B vaccination in all adults (19–59 years).1* As a result, millions of adults are now recommended to be protected against hepatitis B.2
To comply with the policy, we have updated [INSERT UPDATED ALERT] in our EHR system to easily identify patients recommended for vaccination. After viewing the [INSERT UPDATED ALERT], you can add a hepatitis B vaccination order to the patient’s care plan.
In order to increase vaccination rates, please begin identifying all adults recommended for hepatitis B vaccination in accordance with the CDC’s updated universal policy.1*
Sincerely,
[XXX]
*The Centers for Disease Control and Prevention (CDC) recommends hepatitis B vaccination for all adults aged 19 through 59 years and adults 60 years or older with risk factors for hepatitis B. Adults who are 60 years or older without known risk factors may also receive vaccination.1
References: 1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483. 2. Data on file. Dynavax Technologies Corporation; 2022.
US-23-00-00020 | March 2023
Resources For Patient Communication
to your clipboard Templated Email to Patient #1
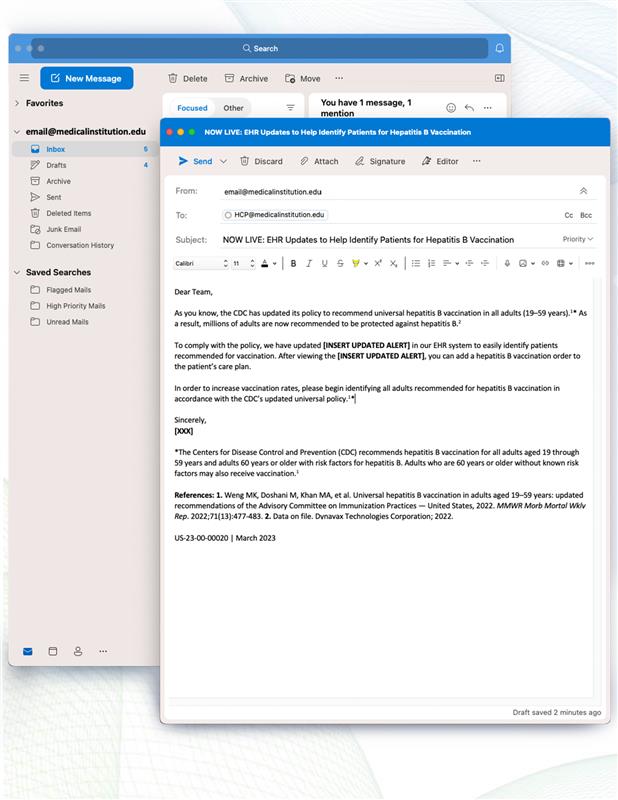
Dear [XXX],
The Centers for Disease Control and Prevention (CDC) now recommends universal adult hepatitis B vaccination1:
- All adults aged 19 through 59 years and adults 60 years or older with risk factors for hepatitis B should receive vaccination.
- Adults who are 60 years or older without known risk factors may also receive the vaccine.
If you are not vaccinated or unsure if you are vaccinated against hepatitis B, please schedule an appointment or speak with a pharmacist at your local retail pharmacy to discuss this new CDC recommendation and/or receive your hepatitis B vaccination.
Sincerely,
[XXX]
Reference: 1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483.
US-23-00-00020 | March 2023
to your clipboard Templated Email to Patient #2
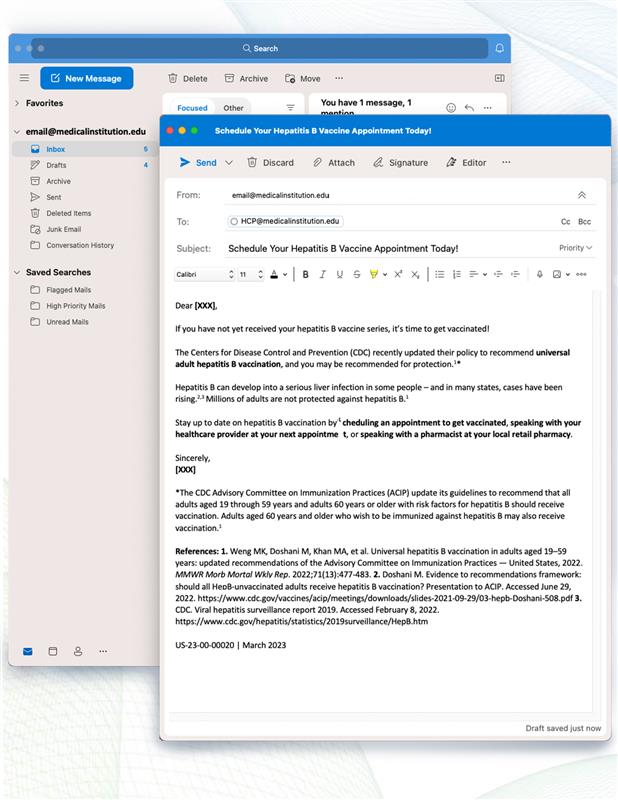
Dear [XXX],
If you have not yet received your hepatitis B vaccine series, it’s time to get vaccinated!
The Centers for Disease Control and Prevention (CDC) recently updated their policy to recommend universal adult hepatitis B vaccination, and you may be recommended for protection.1*
Hepatitis B can develop into a serious liver infection in some people – and in many states, cases have been rising.2,3 Millions of adults are not protected against hepatitis B.1
Stay up to date on hepatitis B vaccination by scheduling an appointment to get vaccinated, speaking with your healthcare provider at your next appointment, or speaking with a pharmacist at your local retail pharmacy.
Sincerely,
[XXX]
*The CDC Advisory Committee on Immunization Practices (ACIP) update its guidelines to recommend that all adults aged 19 through 59 years and adults 60 years or older with risk factors for hepatitis B should receive vaccination. Adults aged 60 years and older who wish to be immunized against hepatitis B may also receive vaccination.1
References: 1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483. 2. Doshani M. Evidence to recommendations framework: should all HepB-unvaccinated adults receive hepatitis B vaccination? Presentation to ACIP. Accessed June 29, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-09-29/03-hepb-Doshani-508.pdf 3. CDC. Viral hepatitis surveillance report 2019. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
US-23-00-00020 | March 2023
to your clipboard Patient Chart Message Template
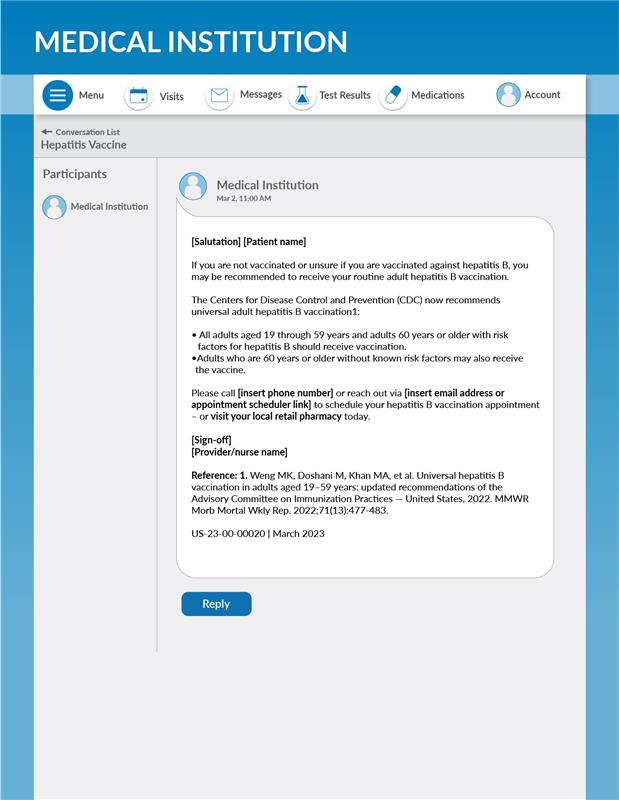
[Salutation] [Patient name]
If you are not vaccinated or unsure if you are vaccinated against hepatitis B, you may be recommended to receive your routine adult hepatitis B vaccination.
The Centers for Disease Control and Prevention (CDC) now recommends universal adult hepatitis B vaccination1:
All adults aged 19 through 59 years and adults 60 years or older with risk factors for hepatitis B should receive vaccination.
Adults who are 60 years or older without known risk factors may also receive the vaccine.
Please call [insert phone number] or reach out via [insert email address or appointment scheduler link] to schedule your hepatitis B vaccination appointment – or visit your local retail pharmacy today.
[Sign-off]
[Provider/nurse name]
Reference: 1. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483.
US-23-00-00020 | March 2023
CDC, Centers for Disease Control and Prevention; EHR, electronic health record.